- E-Shop
-
Labside
- NOBILDENT Delux EPN Artificial Denture Teeth
- NOBILDENT Prime DXL Artificial Denture Teeth
- NOBILDENT Select XL Artificial Denture Teeth
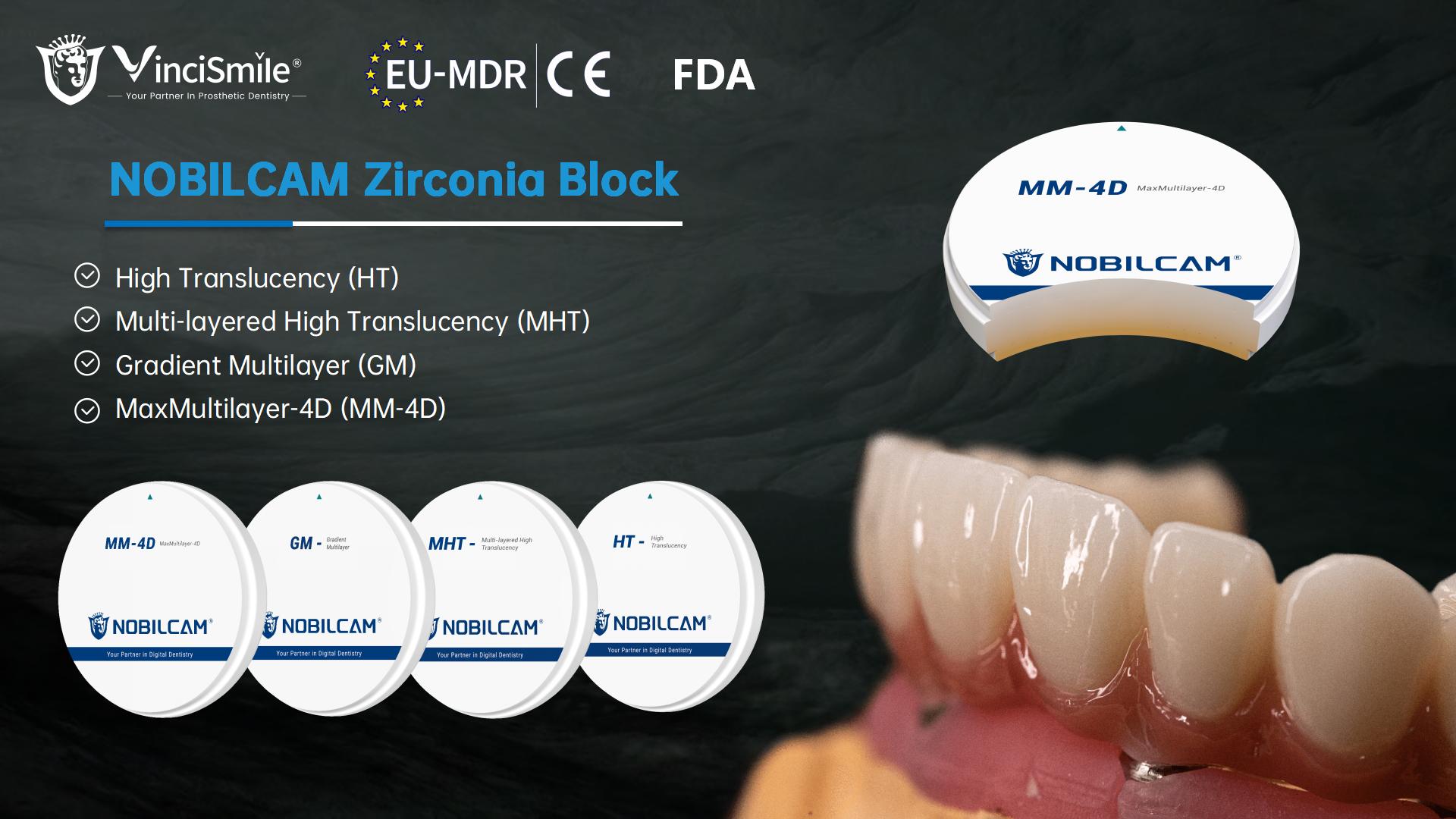
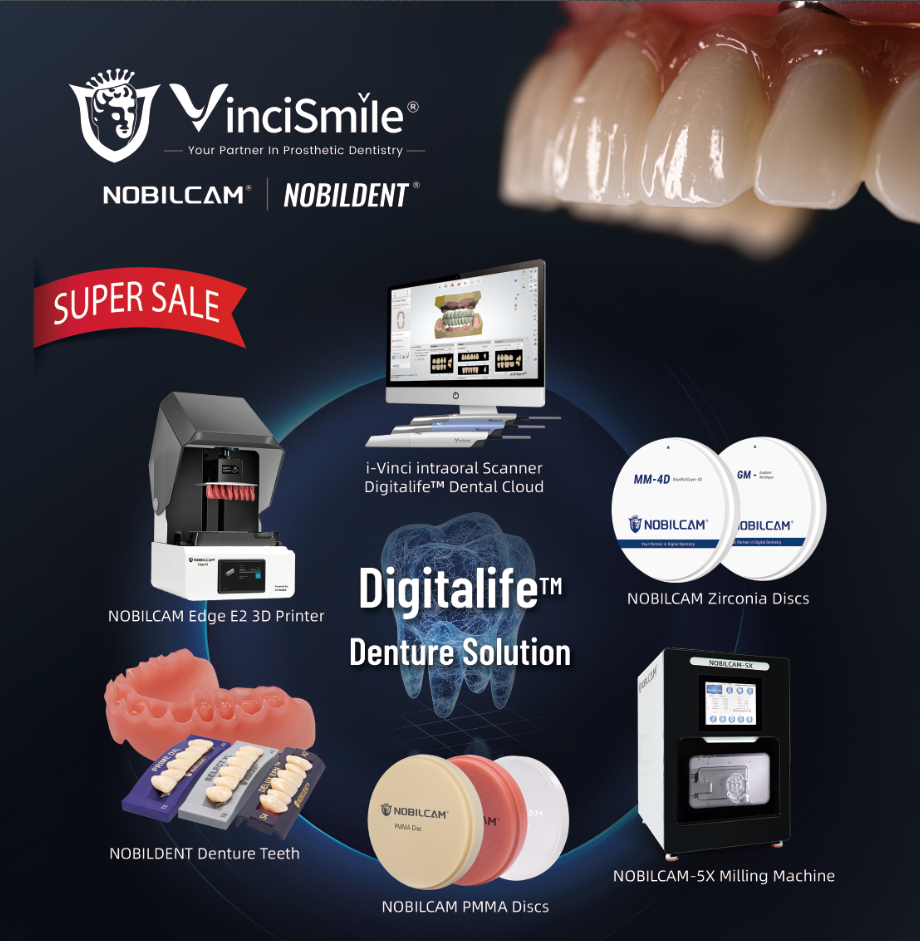
- NOBILCAM MM-4D MaxMultilayer Zirconia Discs
- NOBILCAM GM-3D Gradient Multilayer Zirconia Discs
- NOBILCAM MHT-Multilayer High Translucent Zirconia Discs
- NOBILCAM HS-High Strength Zirconia Discs
- NOBILCAM HT-High Translucent Zirconia Discs
- NOBILCAM AT-Anterior Translucent Zirconia Discs
- NOBILCAM Multilayer PMMA Discs
- NOBILCAM Flexible Discs
- NOBILCAM Monolayer PMMA Discs
- NOBILCAM Clear PMMA Discs
- NOBILCAM Pink PMMA Discs
-
Chairside
- PERFIT Putty
- PERFIT Heavy Body
- PERFIT Regular Body
- PERFIT Light Body
- PERFIT A-Silicone for Bite Registration
- PERFIT Tray Adhesive
- Dental Impression Kit
- HugeBond Universal FliPro Light Cure Dental Adhesive
- HugeBond Universal Light Cure Dental Adhesive
- P-Etchant Phosphoric Acid Etching Gel
- HF-Etchant Hydrofluoric Acid Etching Gel
- TopCEM-Ceramic Primer Ceramic Coupling Agent
- TopCEM Dual Cure Resin Cement
- TopCEM Elite System
- TopCEM-Veneer Light Cure Veneer Cement
- TopCEM-Try In Veneer Try-in Gel
- TopCEM Veneer System
- TopCEM Resin Modified Glass Ionomer Cement-Luting
- TopCEM Vigor SA Self-Adhesive Resin Cement
- TrusFIL Universal Composite Restorative
- TrusFIL-Flow Flowable Composite Restorative
- TrusFIL X-Blend Universal Composite Restorative
- TrusFIL Restoration System
- UltraCore Core Build-up Material
- Proseal F Enamel Coating Resin
- Pro Shield Fluor Protector
- Dental Equipment
- Solution
- Company
-
Contact Us
 en
en  ja
ja  fr
fr  de
de  es
es  ru
ru  ar
ar  th
th  vi
vi  id
id  hi
hi